The Issue
Radiation exposure from a radiological/nuclear incident, including accidents, acts of terrorism, and nuclear reactor events could result in mass casualties and expose victims to substantial doses of ionizing radiation. These events create challenges for initial responders to effectively triage casualties and assess radiological exposures, further complicated by biological radiation absorption characteristics. Depending on the absorbed dose, the biological intensity and onset/latency of Acute Radiation Syndromes (ARS) and Delayed Effects of Acute Radiation Exposure (DEARE) vary. ARS follows a deterministic path whereby dose effects have distinct clinical outcomes. Therefore, early detection of the extent of radiation injury is critical to ensure timely administration of countermeasures and proper allocation of the available resources necessary to save lives.
Studies have identified a panel of candidate radio-responsive serum proteins and messenger RNAs in peripheral blood; however, tests that measure these responses do not appear robust across a broad dose range and their sensitivity, accuracy, and rapidity are not adequate for use in triage for a mass casualty event. Inherent issues such as the depletion of lymphocytes, differences in DNA damage response, and gene variation in stress response sensitivity confound normalization and radiation dose reconstruction readings from these messenger RNA panels.
Current approaches for radiation biodosimetry rely on clinical symptoms, evaluation of lymphocyte depletion kinetics, as well as dicentric chromosome assay (DCA) assessment. Unfortunately, the DCA requires processing in a specialized laboratory and a three to four-day analysis time. Patients who receive high doses of radiation usually require management decisions well before DCA results are available. With limited national laboratory capacity and a small but critical window to begin patient treatment, a biodosimetry solution capable of overcoming these challenges in the initial phase of a large-scale incident is vital to emergency preparedness.
Our Product
To address the critical need, Capture is productizing the radiation biodosimetry diagnostic test MiRAD™; an innovative High-Throughput (HT), microRNA (miRNA) biomarker-based biodosimetry assay which will enable individualized clinical biomarker quantification in direct correlation with radiation exposure.
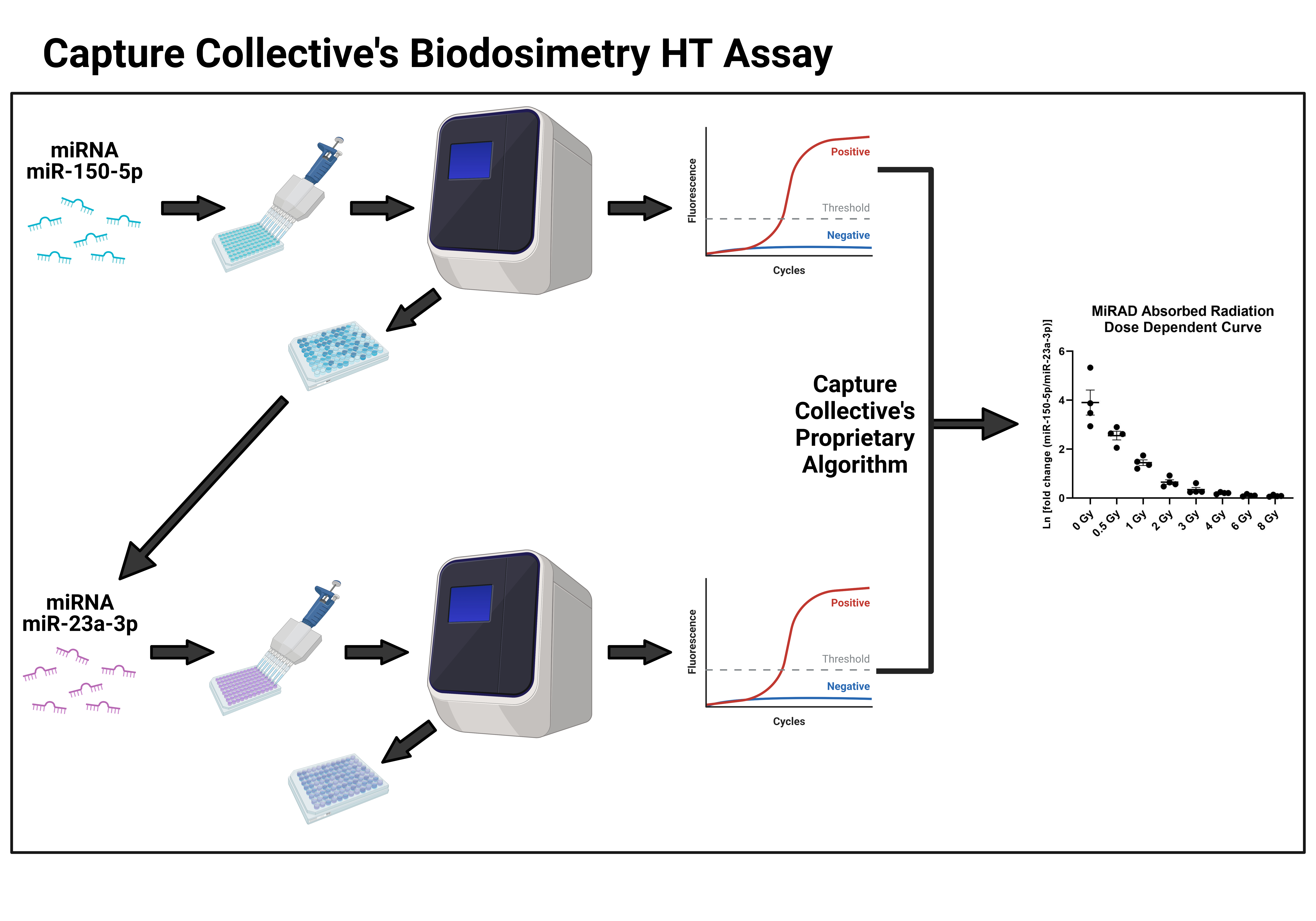
MiRAD™ is superior to most existing Biodosimetry assays in terms of:
- Minimally Invasive
- Sample Volume: 1-2 drops of blood (normalization is not volume/quantity dependent)
- Broad Analytical Range: Analyte stable from hours to days
- Robustness: Response in males and females, young to elderly
- Rapidity: Short processing time (~5 hours)
- Ease of Performance: Can be done in a reasonably equipped molecular biology laboratory
The MiRAD™ assay will provide qualitative and quantitative information to assist first responders in making informed triage, treatment, and recovery decisions in the event of a radiological disaster. Currently, there are no HT biodosimetry assays approved by the FDA for human use but the characteristics and performance of the MiRAD™ assay align with the FDA’s guidelines for developing a radiation dosimetry assay following triage and follow-up after radiological events.
Contact Us
To learn more about Capture and the MiRAD™ assay, fill out this form and we will get back to you.
